Home > Downloads > Articles > Culture of Small Zooplankters for the Feeding of Larval Fish
Culture of Small Zooplankters for the Feeding of Larval Fish
October 2000
Southern Regional Aquaculture Center
Granvil D. Treece1 and D. Allen Davis2
Read the full article at Southern Regional Aquaculture Center site.
In nature, zooplankton is one of the primary foods of larval fish. Two of the dominant zooplankton groups are Rotifera (rotifers) and a sub-class of the Crustacea, Copepoda (copepods). These two groups are the preferred prey for shrimp and fish and are the live feeds used most often by culturists. The intensive larval culture of most marine fish depends on a large supply of zooplankton.
Brachionus plicatilis (Fig.1a), is a small rotifer first developed as larval fish food in Japan in the 1950s. Since then, many methods of culturing it have been developed. More than 60 species of marine finfish are cultured using B. plicatilis as live food. This publication will concentrate on the culture and feeding of rotifers, but will include information on less used zooplankton such as cladocerans (water fleas), copepods and tintinnid ciliates. An important larger zooplanktor used in aquaculture is the Artemia (brine shrimp), which is the subject of SRAC publication 702.
Rotifers
B. plicatilis is the species used most commonly to feed larval fish in hatcheries around the world. It is a euryhaline species, small and slow swimming, with good nutritional value. It is well suited to mass culture because it is prolific and tolerates a wide variety of environmental conditions.
Strain selection is important because reproduction rate, size and optimum culture conditions (temperature and salinity) can all vary with different strains and species. Some freshwater rotifer variation can be seen in Figure 1b. Two of the best known strains of brackishwater rotifers were thought to be morphotypes of B. plicatilis, and were referred to as the "large" (L) and "small" (S) types. Later it was found that these are two different species (L being B. plicatilis and S being B. rotundiformis). Mean dry weights are approximately 0.33 microgram/rotifer for the L type and 0.22 microgram/rotifer for the S type. The size of the S type is 126 to 172 micrometers according to one source, and 100 to 340 micrometers according to another. The L type is 183 to 233 micrometers according to one source, and 130 to 340 micrometers according to another. Larval fish survive better with L-type rotifers, probably because the larvae use less energy to feed on larger rotifers.
Rotifers may tolerate 1 to 97 ppt salinity, but optimum reproducreproduction occurs below 35 ppt. Most production facilities use 10 to 20 ppt salinity. Abrupt salinity changes of more than 5 ppt can inhibit swimming or even cause death, so acclimation should be done slowly and carefully.
Temperature, salinity and feed concentration all affect the growth rate of rotifers, but temperature is the most critical factor. The optimum temperature for most strains is 28 to 32 °C (82.4 to 89.6 °F). Above 28 °C, the salinity and size of the strain are not very critical, but the density of feed is very important. Below 26 to 28 °C (78.8 to 82.4 °F), the bigger strains tend to grow faster than the smaller ones.
Rotifers have broad nutritional
requirements that must be met to
produce stable cultures. They are
planktonic filter feeders, feeding
on organic particles brought to
their mouths by the movements of
their coronas. The corona is a ciliated
organ on the head region that
characterizes rotifers and is their
means of locomotion. Rotifers
ingest many types of feed, including
bacteria, as long as the size of
the particle is appropriate, so a
variety of food sources can be
used to rear rotifers. However,
rotifers cultured indoors often
require vitamin B12 and vitamin A
supplements.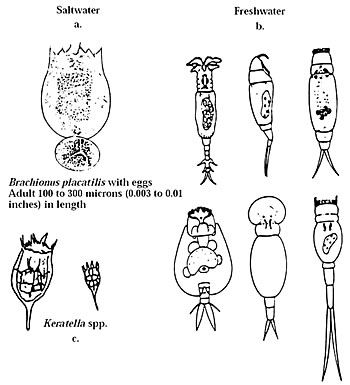
The nutritional value of rotifers for larval fish depends on the rotifers' food source. Researchers have determined that highly unsaturated fatty acids (HUFAs) are essential for the survival and growth of marine finfish larvae. Rotifer feeds containing DHA, 22:6n-3, docosahexaenoic acid, and EPA, 20:5n-3, eicosapentaenoic acid, can be valuable, with DHA the more essential for marine fish larvae. Depending upon their food source, rotifers are about 52 to 59 percent protein, up to 13 percent fat, and 3.1 percent n-3 HUFA.
There are many methods of culturing rotifers. Some are low-density and some high-density. An early method involved daily transfers of rotifers to fresh tanks of the same size after most of the algae were consumed. Following this, batch, semi-continuous, continuous and feedback culture techniques evolved. Each system has advantages and disadvantages. Batch culture is the most reliable but the least efficient. Semi-continuous is less reliable than batch but more efficient; however, it allows wastes to build up, which causes contamination. Continuous cultures are the most efficient and consistent but are maintained under strictly defined conditions and are almost always "closed" and indoors, which limits the size and increases the cost of the operation. The feedback system, developed in Japan, uses wastes from rotifer culture (treated by bacteria and the nutrients retrieved) as fertilizer for algae cultured in a separate tank. The Japanese consider this method the most efficient and reliable. The culture technique described in this publication is usually referred to as "semi-continuous" or the combined "batch/semi-continuous technique."
Nutrient sources for culturing rotifers include baker's yeast and emulsified oils; algae (Isochrysis galbana), yeast and emulsified oil; algae alone; bacteria alone; and outdoor culture using semi-pure or wild strains of algae. The highest reproduction rate (21 offspring per female every week) has occurred when rotifers were fed a pure diet of Isochrysis galbana (Tahiti strain) and kept at a temperature of 20 to 21 °C (68 to 69.8 °F). The optimum feeding rate is 105 to 107 cells of the algae Nannochloropsis oculata per individual rotifer, or 106 to 107 cells of baker's yeast per individual rotifer. The normal concentration of rotifers is about 100 to 200 per ml, but often reaches more than 1,000 per ml with an adequate food supply. And if there is also a pure oxygen supply instead of aeration, the number will reach more than 10,000 individuals per ml. Concentrated Chlorella sp. also can be used for rotifer culture. No one food source contains all the nutrients required for the longterm culture of a species. Several food sources should be used for cultures that are to be maintained for long periods of time.
Read the rest of the article at SRAC web site.
1 Texas A&M University, Sea Grant College Program
2 Auburn University

Exceptional Aquaculture
Reliable Results
4569 Samuel Street
Sarasota, FL 34233
v: 1-800-889-0384
f: (941) 922-3874
e: sales@mblaquaculture.com
www.mblaquaculture.com

